Title
Author
DOI
Article Type
Special Issue
Volume
Issue
Journal Info
Special Issues
Acute Trauma: Immediate Interventions, Long-Term Impacts, Advancements, and Medico-legal Evaluation
Editor: Prof. Dr. Giuseppe Basile, MD,Dr. Luca Bianco Prevot, MD,Dr. Vittorio Bolcato, MD
Submission deadline: 31 July 2026
Advances in Emergency Cardiac Care
Editor: Prof. Dr. Ozgur Karcioglu, MD, FEMAT,Assoc. Prof. Dr. Canan Akman, MD
Submission deadline: 01 March 2026
Advances in the Diagnosis and Management of Sepsis and Septic Shock
Editor: Prof. Dr. Ozgur Karcioglu, MD,Assoc. Prof. Dr. Feride Sinem AKGUN, MD,Assist. Prof. Dr. Betul AKBUGA OZEL, MD
Submission deadline: 01 May 2026
2025 Annual Meeting of Taiwanese Society of Regional Anesthesia & Pain Management (TSRA-PM) in Conjunction with International Symposium of Regional Anesthesia Asia (RA Asia)
Editor: Assoc. Prof. Dr. Jui-An Lin, MD, PhD,Assist. Prof. Dr. Kuo-Chuan Hung, MD,Enoch Yi-No Kang,Prof. Dr. Ke-Vin Chang, MD, PhD, CIPS, RMSK, ASRA-PMUC,Assoc. Prof. Dr. Wu Shao Chun, MD,Dr. Shang-Ru Yeoh, MD, MSc
Submission deadline: 01 August 2026
Critical Interventions: Enhancing Emergency Medical Services through Interdisciplinary Research
Editor: Prof. Dr. Krzysztof Goniewicz, PhD,Prof. Dr. Ahmed M. Al-Wathinani, PhD
Submission deadline: 28 February 2025
Advances in Obstetric Anaesthesia
Editor: Prof. Tatjana Stopar Pintarič, MD, PhD, DEAA
Submission deadline: 31 December 2023
Cardiothoracic anaesthesia and intensive care: current challenges and novelties
Editor: Dr. Marko Zdravkovic, MD, PhD
Submission deadline: 30 November 2022
Selected papers from Korean Society of Emergency Medicine (KSEM) in 2021-2022
Editor: Kyungwon Lee, MD, PhD,Young Duck Cho, MD, PhD
Submission deadline: 31 December 2022
Editorial Process
A summary of the editorial process is given in the flowchart below.
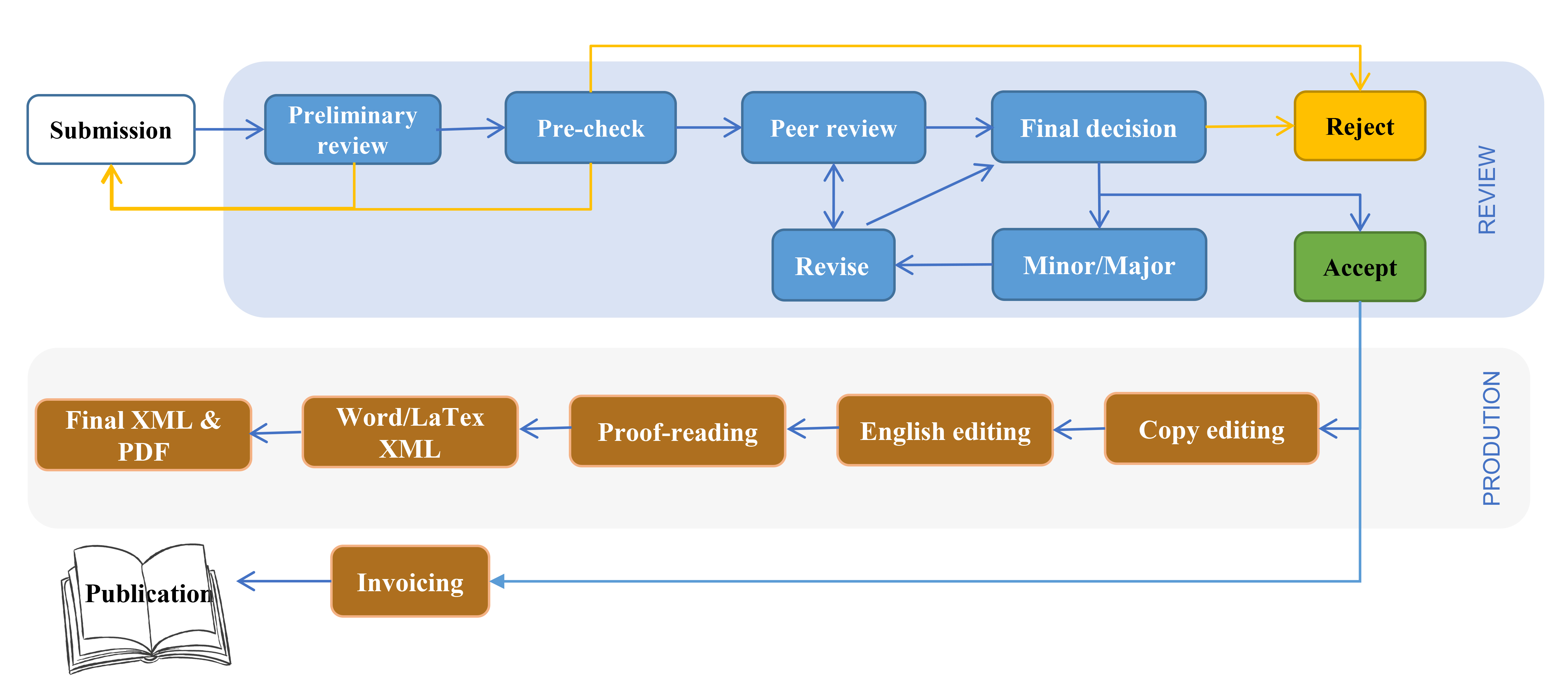
Signa Vitae Editorial Process
The following provides notes on each step.
2. Preliminary review/Pre-check
Immediately after submission, a Preliminary review will first be carried out by the managing editor. The relevant editor, i.e, the Editor-in-Chief in the case of regular submissions, or the Guest Editor in the case of Special issue submission, or an Editorial Board member in case of a conflict of interest and of regular submissions if the Editor-in-Chief allows, will be notified of the submission and invited to perform a Pre-check. The relevant editors can decide to continue with the peer-review process, reject a manuscript, or request revisions before peer-review.
Signa Vitae (SV) requires that editorial staff or editors not be involved in processing their own academic work. The Editor or members of the Editorial Board may occasionally submit their own manuscripts for possible publication in the journal. In these cases, the peer review process will be managed by alternative members of the Board. Submissions will be assigned to at least two independent outside reviewers. The submitting Editor/Board member will have no involvement in the decision-making process. Decisions will be made by other Editorial Board Members who do not have a conflict of interest with the author.
Guest Editors should not hold conflicts of interest with authors whose work they are assessing (e.g., from the same institution or collaborate closely). In this case, the Editor-in-Chief or a suitable Editorial Board member will make the Pre-check and Final decisions for submitted papers.
3. Peer-review
Signa Vitae adopts double-blind peer review. Once a manuscript passes the initial checks, it will be assigned to at least two independent experts for peer-review. SV editors will check to make sure there are no conflicts of interest before contacting reviewers, and will not consider those with competing interests. Reviewers are asked to declare any conflicts of interest before reviewing any submitted manuscript. For more details, see our Peer Review Policy.
4. Author Revision
In cases Minor revisions/Major revisions are recommended, the author is usually requested to revise the paper before referring to the the relevant editor. Articles may or may not be sent to reviewers after minor revision, which depends on whether the reviewer requested to see the revised version or not. The revised manuscript after major revision will be sent back to reviewers again. We typically allow no more than two rounds of major revisions per manuscript. Any further revision needed will follow the decision of the relevant editors.
All reviewer comments should be responded point-by-point. Where the authors disagree with a reviewer, they must provide a clear response or rebuttal.
5. Editor Decision
The acceptance of the manuscript will depend on the revisions made to the manuscript. Authors need to provide a point by point response or a rebuttal if some of the reviewer’s comments cannot be revised. The Acceptance or rejection decision will be made by the relevant editor after peer review, and the relevant editor can select from the following options: Accept in Present Form, Accept after Minor Revisions, Reconsider after Major Revisions, Reject.
(In some instances, the relevant editor may support a decision of manuscript acceptance despite a reviewer recommendation to reject. Journal staff will seek a second independent opinion from an Editorial Board member or the Editor-in-Chief before communicating a final decision to the authors.)
6. Author Appeals
Authors may appeal a rejection judgement by sending an e-mail to the journal’s Editorial Office. The appeal must provide a detailed justification, including point-by-point responses to the reviewers' and/or Editor's comments. The Managing Editor of the journal will forward the manuscript and related information (including the identities of the referees) to the Editor-in-Chief, Associate Editor, or Editorial Board member. The Editor being consulted will subsequently be asked to provide an advisory recommendation on the manuscript and may recommend acceptance, further peer-review, or uphold the original rejection decision. A reject decision at this stage is final and cannot be reversed.
7. Production
SV carries out production on all manuscripts, including copy editing, layout and conversion to XML. Language editing is carried out by professional English editing editors. We recommend authors use Englishgo’s English editing service prior to publication or during revisions. If you have used an alternative service, please provide a copy certificate to the Editorial Office.
The submitted, accepted and published dates will be shown in the PDF and XML files of the published articles, a digital object identifier (DOI) number will also be assigned for each published article. The submitted date is the date on which the editors received the original (or if previously rejected, the resubmitted) manuscript. The accepted date is when the editor sends the acceptance letter. The published date is the earliest date that the final version-of-record is made available on the publisher's website.
8. Publishing Standards and Report Guidelines
SV follows the following guidelines and standards. Submission of a manuscript to Signa Vitae implies that all authors have read and agreed to its content, and the manuscript conforms to the journal’s policies.
ICMJE: Medically related SV follows the recommendations of the International Committee of Medical Journal Editors. The guidelines comprehensively cover all aspects of editing, from how the journal is managed to details about peer review and handling complaints.
CONSORT statement covers reporting of randomized, controlled trials. We encourage authors to verify their work against the checklist and flow diagram and upload them with their submission.
PRISMA covers systematic reviews and meta-analyses of interventional studies and MOOSE for systematic reviews and meta-analyses of observational studies. Authors are recommended to complete the checklist and flow diagram and include them with their submission.
ARRIVE contains guidelines for reporting in vivo experiments. Authors are recommended to verify their work against the checklist and include it with their submission.
STROBE guidelines cover reporting of observational studies. Also, for diagnostic studies, authors are encouraged to use STARD guideline.
CARE guideline (for CAse REports) was developed by an international group of experts to support an increase in the accuracy, transparency, and usefulness of case reports. View and download the CARE checklist.
Other related guidelines, please visit the EQUATOR network (https://www.equator-network.org/).
9. Ethical Standards
SV follows the guidelines of the Committee on Publication Ethics (COPE) and the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
SV follows COPE’s procedures for dealing with potentially unethical behavior by authors, reviewers or editors. Our editorial staff are trained in how to detect and respond to potential ethical problems.
More information about our Publishing Ethics policy.
10. Editorial Independence
Editorial independence dictates that the decision to accept or reject a manuscript is based on the scientific merit of the article but not to any other relations for example pressure from the publisher to the journal editor. This means that Editor is independent in his/her decision and will not be under pressure of any influential body or organization.
Our editorial policy is consistent with the principles of editorial independence presented by the World Association of Medical Editors (WAME).
Updated: January 03, 2025
Abstracted / indexed in
Science Citation Index Expanded (SCIE) (On Hold)
Chemical Abstracts Service Source Index
Scopus: CiteScore 1.3 (2024)
Embase
Submission Turnaround Time
Conferences
Top
